Minimum custom amount to enter is AED 2
By donating, you agree to the Privacy Policy and Terms of Service

AstraZeneca Oxford announced recently for the first time that its coronavirus vaccine could have rare and unprecedented side effects.
The company was recently hit with a class action, with the plaintiffs claiming that the vaccine- which was developed at Oxford University, was responsible for deaths and tens of serious injuries.
According to the report, 51 families contributed to the class action, suing the AstraZeneca Oxford for GBP 100 Million, and holding the company responsible for the harmful side effects.
In response to the statement, the Director of Vaccinations at the Egyptian Vaccine Authority Moustafa Al Mohammadi explained in a statement that “The vaccine were given on emergency approval, and that everyone received them at their own responsibility after signining a consent form.”
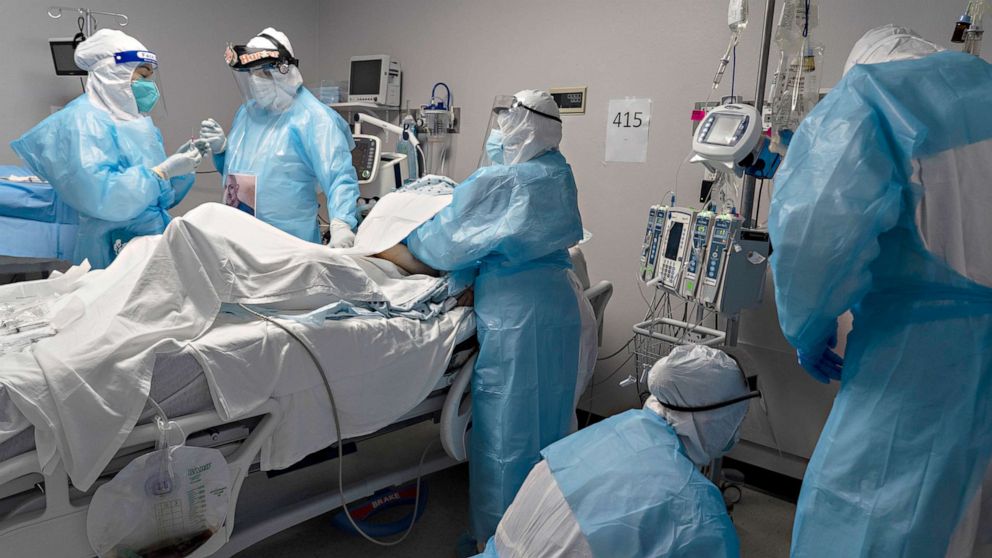
Al Mohamadi also indicated that the vaccine is still used in Egypt and that he doesn’t have information about the rare side effects.
He also denied stopping its use, or conducting a recent review of vaccinating citizens with the vaccine used since the first preventative wave of vaccine was first launched.
AstraZeneca said in a press release that in very rare cases, the vaccine could cause what is known as blood clots with thrombocytopenia syndrome- known as TTS, low platelet count, menstural disturbances, heart complications, and even death.
View this post on Instagram
Next:Explosive Showdown… Salah And Klopp Ignite In Fiery Clash During West Ham Match
Minimum custom amount to enter is AED 2
By donating, you agree to the Privacy Policy and Terms of Service